Our services
More than a traditional OEM/ODM partner
With a proven track record in end-to-end innovation, we advance the science, confirm it through advanced simulation, and clinically validate our technologies and solutions. In parallel, we explore new applications – all in the context of addressing unmet consumer needs.
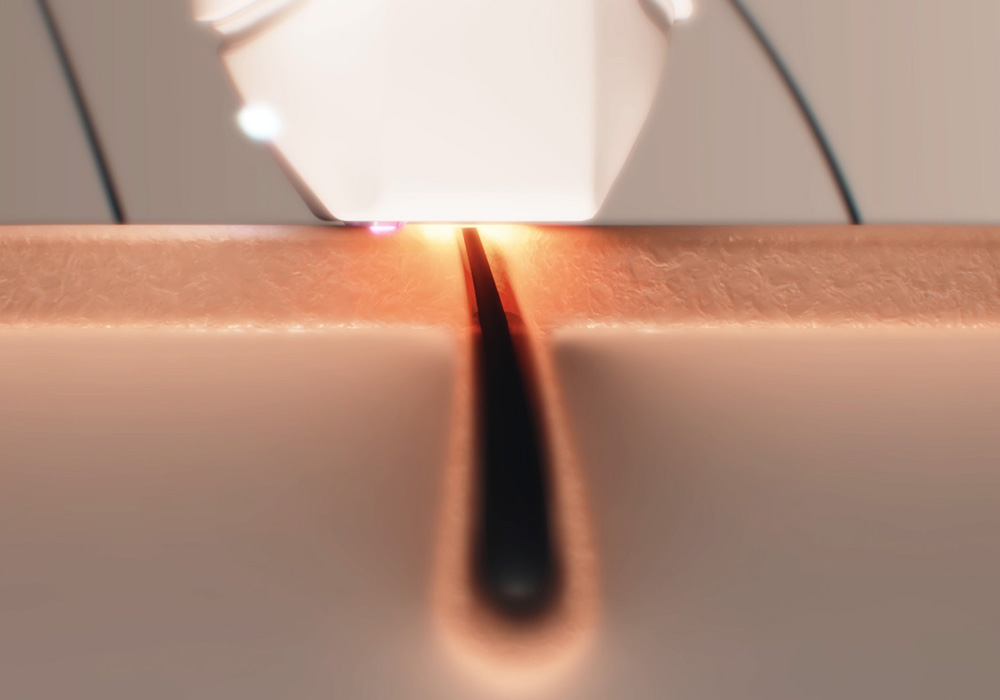
Leaders in energy-based innovation for skin and hair
We specialise in understanding the science of how energy interacts with skin and hair – developing breakthrough solutions grounded in research, validated by evidence, and trusted by industry leaders.
Research & simulation
Accelerate product development with proven precision
Enhance your R&D capabilities with our proprietary simulation modelling. Designed to predict the performance of both existing and new systems, our platform streamlines development cycles and reduces costly trial-and-error. Every model is backed by clinical validation – ensuring confidence in outcomes before market launch.

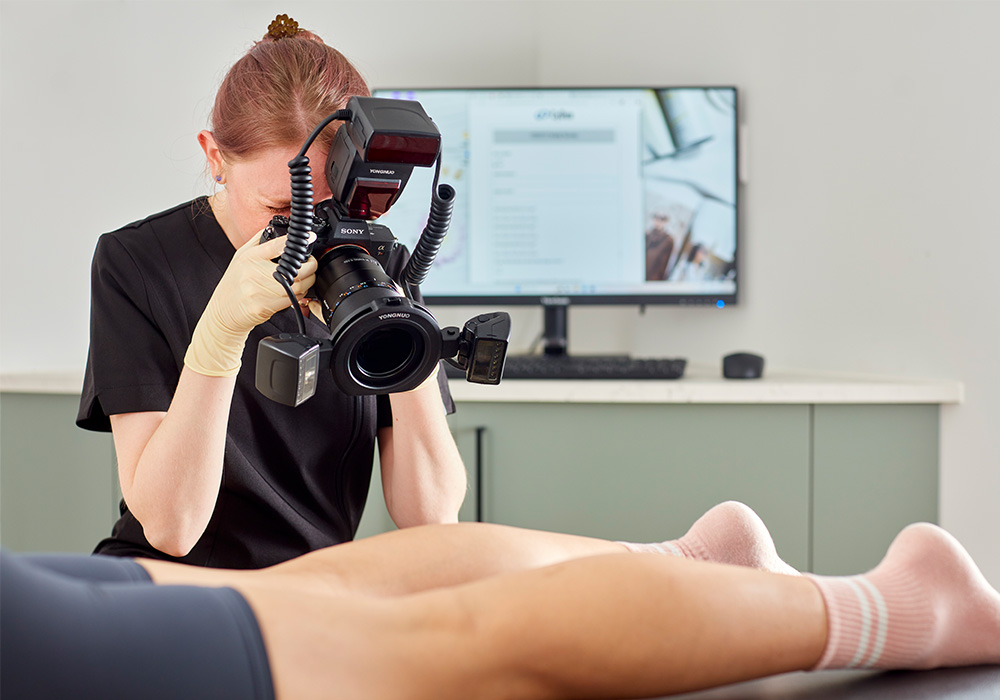
Clinical expertise
Evidence-backed innovation through in-house clinical excellence
Development expertise
Streamlining product innovation from concept to market
Our in-house teams specialise in the rapid development of high-volume beauty technology products. From upstream platform technology exploration to prototyping and full-scale manufacturing, we deliver market-ready solutions with efficiency and precision.

Key capabilities:
In-house testing
laboratory
Ensuring rigorous quality and performance validation (Ocular safety, spectral analysis, EMC, environmental and reliability testing under one roof.)
Embedded software engineering
Compliant with EN 60335-1, EN 60601-1 and EN 62304 standards.
Comprehensive regulatory approval management
Full support throughout the approval process.
International medical and regulatory compliance
Meeting the highest global standards. Compliant with ICH-GCP, ISO 14155 and ISO 62366.
Global regulatory expertise
Navigating complexity with proven compliance leadership
Our dedicated regulatory teams bring deep expertise in medical device approvals and global safety standards. From developing regulatory strategies and determining device classifications to preparing technical documentation and managing post-market surveillance, we have a strong track record of securing clearances from the FDA, EU MDR, and China NMPA. We ensure your products achieve the highest levels of compliance – efficiently and without unnecessary delays.





Global manufacturing capabilities at scale
Precision manufacturing for regulated markets
With facilities in the UK and China, we specialise in high-volume manufacturing tailored to the needs of highly regulated medical and beauty markets. Producing over 4 million units annually, our operations are built for efficiency, compliance, and scale.
Expertise in medical device GMP
Adhering to globally recognised manufacturing practices
Fully compliant with international regulatory standards
Supporting market access worldwide
Ongoing quality optimisation
Embedded continuous improvement within strict regulatory frameworks

